Keywords:
Caplacizumab-YHDP, Caplacizumab-YHDP’s R&D Progress, Mechanism of Action for Caplacizumab-YHDP, drug target for Caplacizumab-YHDP.
Description:
This article summarized the latest R&D progress of Caplacizumab-YHDP, the Mechanism of Action for Caplacizumab-YHDP, and the drug target R&D trends for Caplacizumab-YHDP.
Text:
Caplacizumab-YHDP‘s R&D Progress
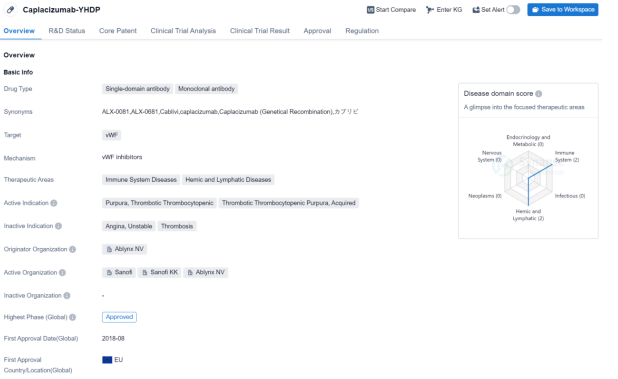
Caplacizumab-YHDP is a drug classified as a single-domain antibody and monoclonal antibody. It targets vWF (von Willebrand factor) and is primarily used in the treatment of immune system diseases and hemic and lymphatic diseases. The active indication for Caplacizumab-YHDP is purpura, thrombotic thrombocytopenic (TTP), specifically acquired TTP.
The drug was developed by Ablynx NV, a pharmaceutical company. Caplacizumab-YHDP has reached the highest phase of development. It received its first approval in the European Union in August 2018.
Caplacizumab-YHDP falls under several regulatory designations. It is categorized as an overseas new drug urgently needed in clinical settings, indicating its potential to address unmet medical needs. It also received fast track designation, which expedites the development and review process for drugs that treat serious conditions and fill an unmet medical need. Additionally, Caplacizumab-YHDP is classified as an orphan drug, indicating its intended use in the treatment of rare diseases.
Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Caplacizumab-YHDP: vWF inhibitors
From a biomedical perspective, vWF inhibitors refer to a class of drugs or substances that inhibit the activity of von Willebrand factor (vWF). Von Willebrand factor is a protein that plays a crucial role in blood clotting by helping platelets adhere to damaged blood vessels. However, in certain medical conditions such as von Willebrand disease or thrombotic disorders, excessive vWF activity can lead to abnormal clot formation.
vWF inhibitors work by blocking the binding of vWF to platelets or interfering with its function, thereby preventing or reducing the formation of blood clots. These inhibitors can be used in the treatment and prevention of various conditions associated with excessive clotting, such as deep vein thrombosis, pulmonary embolism, and arterial thrombosis.
It’s important to note that vWF inhibitors should be used under medical supervision, as they can increase the risk of bleeding. The specific mechanism of action and administration of vWF inhibitors may vary depending on the drug or substance being used.
Drug Target R&D Trends for Caplacizumab-YHDP
vWF, or von Willebrand factor, plays a crucial role in the human body’s blood clotting process. It is a glycoprotein that is primarily produced by endothelial cells and megakaryocytes. vWF acts as a carrier protein for clotting factor VIII, protecting it from degradation and facilitating its binding to platelets at the site of injury. This interaction between vWF and platelets promotes platelet adhesion and aggregation, leading to the formation of a stable blood clot. Additionally, vWF also plays a role in maintaining vascular integrity by mediating platelet adhesion to the damaged endothelium. Any abnormalities or deficiencies in vWF can result in bleeding disorders such as von Willebrand disease.
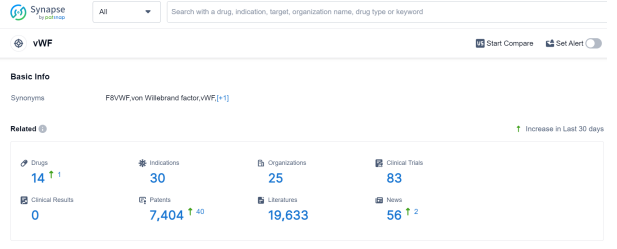
According to Patsnap Synapse, as of 26 Sep 2023, there are a total of 14 vWF drugs worldwide, from 25 organizations, covering 30 indications, and conducting 83 clinical trials.
Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Caplacizumab-YHDP is a single-domain antibody and monoclonal antibody drug that targets vWF. It is primarily used in the treatment of immune system diseases and hemic and lymphatic diseases, specifically acquired TTP. Developed by Ablynx NV, the drug received its first approval in the European Union in August 2018. It has been designated as an overseas new drug urgently needed in clinical settings, fast track, and orphan drug. These designations highlight the drug’s potential to address unmet medical needs and its expedited development and review process.